Effectiveness of Two-Dose Pfizer-BioNTech vaccine among Adolescents in Scotland and Brazil
August 2022: New paper looks at how the effectiveness of two-doses of the Pfizer-BioNTech vaccine changes over time in adolescents. The paper looks at the effectiveness of protection provided against symptomatic infection of the SARS-CoV-2 virus and severe cases of COVID-19.
Vaccine Effectiveness of Two-Dose BNT162b2 Over Time Against COVID-19 Symptomatic Infection and Severe Cases Among Adolescents: Test Negative Design Case Control Studies in Brazil and Scotland
Florentino, P.T.V; Millington, T; Cerqueira-Silva, T; Robertson, C; et. al.
The Lancet Infectious Diseases
Published online: 8 August 2022
Available online at: https://doi.org/10.1016/S1473-3099(22)00451-0
Download the infographic in English
Read the infographic in Portuguese (infográfico em português)
This infographic was created by the EAVE II's Communications & Engagement Officer, Gabriella Linning (Usher Institute, The University of Edinburgh)
Plain English Summary
Cross-country comparison studies provide us with several key strengths over and above individual country studies.
In this case, Scotland and Brazil both have high quality national-scale databases that will make our statistical analyses far more powerful than if done separately.
This reduces confounding in our results. This means that if we see the same or similar patterns happening across two different countries where circumstances are different (e.g. timing of waves of infection, timing of vaccination), we can more safely identify what is causing these patterns to form.
In a similar vein, it minimises the chances of bias relating to factors such as access to health care, occurrence of symptoms, and health seeking behaviour skewing our results.
The Vigivac Project
The VigiVac project is similar to EAVE II. It is Brazil's digital surveillance initiative that seeks to evaluate the effectiveness of vaccines against COVID-19. Our team worked alongside VigiVac's researchers to conduct this piece of research.
Click here to visit the VigiVac website (Portuguese)
VigiVac project is part of Fiocruz, the Oswaldo Cruz Foundation, the most prominent institution of science and technology in health in Latin America.
Visit the Fiocruz website (English)
Visit the Fiocruz website (Español)
Why did we carry out this work?
Currently, little is known about how COVID-19 vaccine effectiveness (VE) changes over time in 12–17-year-olds. This is especially the case with the Omicron variant of coronavirus (SARS-CoV-2), known as B1.1.529.
To date, nobody has reported data about how well young people aged 12-17 are protected against infection and disease after two doses of a COVID-19 vaccine.
Understanding how vaccine protection wanes is important because it can help to inform future vaccine programmes for young people, and potentially prevent further illness and education disruption.
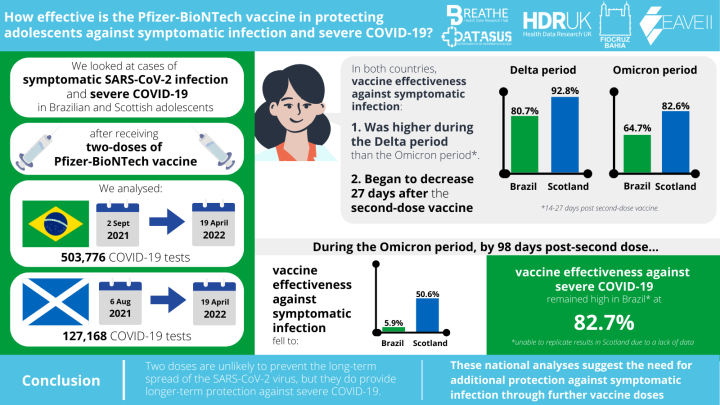
In this study, we looked at symptomatic SARS-CoV-2 infection and severe COVID-19 in 12-17 year-old adolescents in Scotland and Brazil. We studied different time periods after receiving two doses of the Pfizer BioNTech vaccine (BNT162b2), which is the main vaccine licensed for children in this age group.
What data did we use?
We looked at the data of adolescents aged 12-17 years old with COVID-19 related symptoms for the time periods of 2 September 2021 – 19 April 2022 in Brazil, and 6 August 2021 – 19 April 2022 in Scotland.
We did this by linking records of RT-PCR (‘Reverse Transcription-Polymerase Chain Reaction’) and antigen tests for SARS-CoV-2 to national vaccination and clinical records in both countries.
Find out more about: What is PCR?
In the models for both countries we accounted for people’s individual circumstances including:
-
Age
-
Sex
-
Time (week) a person tested positive for infection
-
Location
-
Previous SARS-CoV-2 infection
-
Health conditions that put people at higher COVID-19 risk
In Brazil, we also considered ethnicity, current pregnancy or being in the post-partum period. Unfortunately, we did not have enough data available to consider these factors in our Scottish analysis.
Data from Brazil
To create our Brazilian cohort, we used data about RT-PCR and antigen testing, vaccinations, as well as registered COVID-19 related hospitalisations and deaths
These data sources were linked using information provided by DATASUS, the Health Data Informatics department at the Brazilian Ministry for Health.
Data from Scotland
We gathered our Scottish data through the Early Pandemic Evaluation and Enhanced Surveillance (EAVE II) project, which has been able to link multiple, de-personalised datasets for almost all the Scottish population, in near-real time.
What did we find?
In total, we analysed 503,776 tests from Brazil and 127,168 tests from Scotland.
Vaccine effectiveness against Delta and Omicron variants
We found that vaccine effectiveness against symptomatic COVID-19 varied depending on which variant was the most dominant at a given point in time. Vaccines were less likely to protect during the Omicron period compared to the Delta period.
This was consistent across all time intervals that we examined in both countries: up to 14 days post-first dose, up to 14 days post-second dose and every two-week period thereafter until over 98 days post-second dose.
We found that peak vaccine protection occurred 14-27 days after the second vaccine dose, for both countries and both variants. Vaccine effectiveness was higher in the Delta period than the Omicron period:
-
Delta period: 80.7% in Brazil and 92.8% in Scotland
-
Omicron period: 64.7% in Brazil and 82.6% in Scotland.
Throughout the entire Delta period, VE in Scotland was consistently higher than that in Brazil.
Vaccine effectiveness over time
In both Scotland and Brazil, we found that VE against symptomatic infection began to decline after 27-days post-second dose.
Our results also showed that, after 98 days, the effectiveness of two vaccine doses against symptomatic infection fell to 50.6% in Scotland, and 5.9% in Brazil.
However, after 98 days, vaccine effectiveness against severe disease remained very high, at over 80% in Brazil. An equivalent result could not be made for Scotland due to the small number of severe COVID-19 related cases that occurred overall.
The difference in results for effectiveness against infection and serious disease is most likely to be related to different parts of a person’s immune response, which includes both antibodies and immune cells (e.g. T-cells). The protection of antibodies wanes over time, which increases the risk of infection. However, a person’s T-cell response also protects and limits the progression of COVID-19 and can prevent serious disease.
Why is this important?
To our knowledge, this is the first multi-nationwide study to evaluate how vaccine protection against severe COVID-19 waned for 12–17-year-olds during the Omicron period.
Our results showed that, during the Omicron period, the protection given by the Pfizer- BioNTech vaccine against severe COVID-19 remained strong 98 days after the second dose.
However, vaccine protection against symptomatic infection began to wane 27-days post-second dose.
This suggests that two doses are not enough to continue stopping the spread of the SARS-CoV-2 virus, meaning it is unlikely to prevent further school disruptions, or protect against COVID-19 infection in the long term.
We support the rollout of third and booster dose vaccinations.
Note
This peer-reviewed article builds upon results we previously reported on in a published pre-print.
Click here to read the pre-print
This research is also part of the EAVE II associated project DaC-VaP-2, under its Children and Young People (CYP) theme. This summary can also be found on the DaC-VaP-2 research site.


